News

09 October 2025
2025 Lab Coat Ceremony Welcomes Graduate Students To Their Thesis Labs
An annual tradition marks the start of scientific discovery for the 2024-2025 class of Stowers Graduate School students.
Read Article
News
How much maligned amyloids may be the key to our memories

By Melissa Fryman
Memory. For the majority of us, it is a constant companion, at times uninvited, and at others, cherished. Sometimes we strain to make and retain a fleeting memory, and at others, a memory seems nothing short of automatically installed, enduring a lifetime in our minds.
It’s still mysterious, but the biological process that underlies memory persistence is better understood now than ever, thanks to researchers from the Si Lab at the Stowers Institute.
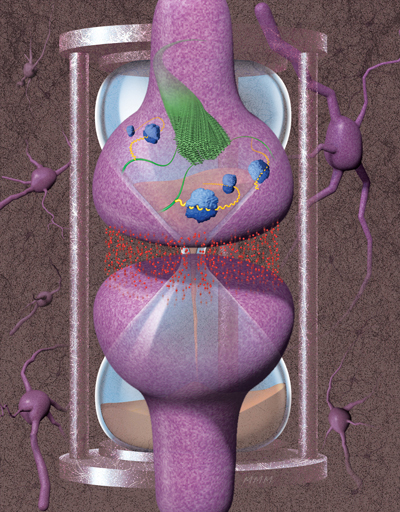
“In a general sense, memory is the ability of a system to experience something and then somehow maintain a signature of that experience, even after the experience has disappeared,” explains Investigator and Associate Scientific Director Kausik Si, PhD. In many biological entities, this capacity is centered in the nervous system, and in particular at synapses, which are the connection points between nerve cells.
Indeed, it was work done on the sensory-motor neural circuit of the sea slug Aplysia californica, by Si and Eric Kandel, MD, at Columbia University nearly twenty years ago, that led to the initial surprising discovery about the molecular requirements for memory maintenance. Their discovery was twofold—an RNA-binding protein called CPEB (cytoplasmic polyadenylation element binding protein) is required for long-term synapse-specific modification, and in order to perform its full function in that capacity, CPEB has to self-assemble to form a functional protein aggregate that can self-renew, effectively acting like a prion.

Kauski Si, PhD, and Ruben Hervas, PhD
Prions are self-renewing protein aggregates, often amyloid aggregates, that have a deservedly frightful reputation. Perhaps the best-known prion in relation to human health is an infectious prion, the hallmark of Creutzfeldt-Jakob disease, which can arise from a genetic origin, or via transmission from an animal, as in the human form of “mad cow” disease.
Amyloids are protein aggregates with characteristic structural features and have an equally bad reputation, being associated with such devastating neurodegenerative pathologies as Alzheimer’s and Parkinson’s diseases, as well as Lewy body dementia. “The central hypothesis is that the protein misfolds and forms an amyloid—either a smaller structure of repeating units or a larger assembly that forms a fibril or fiber—and that it’s a toxic entity,” says Si.
Of their original finding in Aplysia, “that a protein in the nervous system can aggregate in a very specific situation and can be a substrate of something that is stable yet dynamic such as memory—that’s just completely counter to what people were describing,” Si reflects. “Essentially the idea bumped into a hundred years of studies on protein aggregation and amyloids in the brain that suggested one possibility—and then our finding suggested something radically different.”
Over the years, Si and his team have built a strong case for amyloids as a substrate for memory. They showed that CPEB proteins exist in the brains of mice and the fruit fly Drosophila melanogaster, where it is known as Orb2. Importantly, they found that as single units, Orb2 binds to specific protein-encoding mRNA messages, important for memory, located at synapses, acting to repress protein production. As an amyloid assembly, by contrast, Orb2 binds those mRNA messages at synapses and promotes production of those key synaptic proteins, leading to a persistent alternation in synaptic transmission that allows memory persistence. Using memory assays, they have shown not only that self-aggregated Orb2 is required for longterm memory in adult fruit flies but also that already-formed memory is still dependent upon the Orb2 protein.
Most recently, Ruben Hervas, PhD, a postdoctoral researcher in the Si Lab, and collaborators determined the 3D structure of endogenous Orb2 aggregates from fruit fly heads, at near-atomic resolution. Using a technique called cryo-electron microscopy where low-temperature samples (such as small pieces of tissue or purified proteins) are imaged by electron microscopy, followed by computer-assisted reconstruction of the resulting images, they confirmed that, indeed, the state of Orb2 necessary for memory persistence is an amyloid. The implications are vast.
A long history of amyloid research
The term “amyloid” first appeared in the scientific literature in 1838, when it was used to describe the amylaceous, or starch-like, makeup of plants that stained blue when exposed to iodine and sulphuric acid. It wasn’t until 1854 that the term was popularized medically, by the “father of modern pathology” Rudolph Virchow, to describe small white deposits in the brain that stained blue the same way starch did.
“In some sense,” says Si, “from inception, amyloids were associated with the wrong thing—the term was coined to describe something that was supposed to be starch-like, but now we know amyloids are made up of proteins.”
The molecular definition of amyloid emerged from the work of biophysicist William Astbury, continues Si. “He saw that denatured, or unfolded, albumin protein could form fibers in a particular arrangement—layered zigzag-like structures called stacked b-sheets—with a standard distance between the stacked layers forming the b-sheets of 4.7 angstroms.”
A complementary definition of amyloid was derived from the fact that plaques isolated from the brain could be stained with Thioflavin T and Congo Red dyes. “In the 1950s and ‘60s, Alan Cohen and colleagues connected the two. He isolated dye-stained material from the brain, looked at it under an electron microscope, and found that some of the material had similar fibrous appearance.”
Researchers then found that amyloid plaques isolated from the brain were resistant to protein-destroying enzymes and detergents. “This led to the idea that the amyloid state is an unintended consequence of proteins being misfolded or unfolded, shortened, or otherwise degraded or mutated—and that once an amyloid forms, because it’s so stable, it’s an irreversible state that leads to disease. People of course got stuck on this.”
Functional amyloids hit the scene

Atomic structure of biochemically active Orb2 amyloid reveals the stacked three-fold helical symmetry of the filament core.
“In the late 1980s and early 1990s, when yeast fungal prions were discovered, people saw that prions exist in other species, without killing them, and the aggregates of these prions fit the definition of amyloids. But these amyloids were not biochemically active,” Si explains.
“Around 2000, researchers coined the term ‘functional amyloid’ because bacteria or fungi utilize the amyloid to do things—but the protein is not biochemically active. It’s supporting and acting as a stable matrix that provides rigidity, or the ability to stick to things.”
Together, the emergence of functional amyloids in yeast and bacteria suggested the possibility that amyloids could be non-pathogenic. But nobody had ever thought that amyloids could be the substrate of memory.
“They thought I was out of my mind”
“When I thought about it and talked to some people, they thought I was out of my mind. First of all, it’s a prion in the nervous system. Secondly, if it’s forming an amyloid in the nervous system, it cannot be doing anything good. Thirdly, people thought if amyloids are so stable, how can memory be reversible? After all, memory does change and seemingly disappear.”
“I was trained as a physical chemist in India,” Si recounts. “I wanted to switch fields and move to neuroscience, but nobody wanted to take me. So, I actually went to a meeting where I talked to Eric, and I convinced him that he should let me join his group.”
“I was interested in a profound observation that Eric’s group had made. They had discovered if you stop protein synthesis in the synapse, the synapse can still change but it cannot maintain that change over time.”
“That finding suggested that the formation of memory and its subsequent maintenance have distinct requirements,” Si continues. To study how memory sticks around, one must first understand how, and which proteins are made in the synapse.
“When I started out, I never thought it would lead me to prions and amyloids.”
“Magical” protein state
That functional amyloids could exist in the nervous system “provided a very simple solution to one of the most complex biological processes,” says Si. “Our findings suggested that a protein could be used like flipping a switch. By changing the conformation of a protein, the nervous system can encode information on to a stable, yet changeable, physical substrate to achieve something seemingly magical, such as memory.”
“There was a time when people debated,” says Si. “Are there magical genes or magical proteins that the nervous system utilizes to create memory? This discovery suggested there are not, but that instead, there are magical protein states.”
Stuck
The loss of memory can be tragic, and many biomedical efforts are underway toward treating or preventing devastating pathologies such as Alzheimer’s disease and Lewy body dementia. The vast majority of potential therapies are designed to dissolve or prevent the formation of amyloids. Those that have progressed to phase 3 clinical trials have failed to cure memory loss.
“This would imply that the amyloid formation that we see in the nervous system sometimes either is an intended consequence, or, that the mere presence of amyloid is not bad even if its presence may indicate an undesired situation,” says Si.
Scientists in the Si Lab study biological mechanisms underlying the persistence of memory.
Solving for the structure of Orb2
“The very first time I talked with people at Columbia University—some of the most accomplished neurobiologists—they told me, ‘Kausik, the day you solve the structure, come and talk to me.’ They also warned me it wasn’t going to be easy.”
Fast-forward 20 years. In a study published in the March 13, 2020, issue of Science, Si and Hervas have done just that.
“We knew that the Orb2 protein can adopt multiple conformations,” says Si. But, he explains, “often, researchers solve the structure of a protein where it is convenient to get as opposed to where it actually functions.” By expressing a protein in bacteria, for example, “it might form an amyloid, but biochemically it does not function the same way it does in its native context.” Thus, Hervas and Si agreed that although it would be much more difficult, using Orb2 from its original source to solve the structure would be the most meaningful way to proceed.
Because X-ray crystallography—a traditional method for determining protein structures—has limitations when applied to crystallizing large proteins in their native states, solving the structure with that method was unlikely, according to Hervas. And because they could harness the explosive improvements in imaging technology of recent years, they knew that cryo-EM could be the perfect tool.
Enter the collaborators
“This was a really challenging problem, and it exemplifies what I view as the future of science, which is team science,” says James Fitzpatrick, PhD, professor and scientific director of the Washington University Center for Cellular Imaging (WUCCI).
The work is a result of “people with backgrounds in different fields—computational biology, wet lab biology, neuroscience, physics, microscopy, and structural biology—coming together to do this kind of cryo-EM,” says Fitzpatrick.
Leading up to the collaboration, WUCCI at the Washington University School of Medicine in St. Louis had acquired a powerful cryo-EM. Fitzpatrick recounts, “I felt it was important, as there weren’t many of these instruments at all in the Midwest, and particularly in Missouri, that we should try to reach out and engage our regional community. I had gone to Stowers to give a seminar,” Fitzpatrick added. “I love to visit Stowers—it’s such fun.”
Although Fitzpatrick studies pathological amyloids, he admits, “this is the first time I had heard the term ‘functional amyloid.’ Having spoken with Kausik, he had talked about functional amyloids, and he told me he was very interested in cryo-EM. Afterward, I did a bit of reading and I got really excited about this. I told him to send Ruben over for a visit.”
After transporting themselves and their samples by car and train back and forth across the state, Hervas and members of the Si Lab, with the help of Fitzpatrick, developed a process to seamlessly prepare, transport, and then cryogenically freeze and image the samples using the machine at the WUCCI.
It was during this time, Fitzpatrick says, “a cryo-EM legend gave a talk at Stowers.”
The legend, the matchmaker
When Hervas told Eva Nogales, PhD, professor at the University of California, Berkeley, senior scientist at the Lawrence Berkeley National Laboratory, and Howard Hughes Medical Institute investigator, about his interest in using cryoEM to solve the structure of the Orb2 amyloid, she said, “I know exactly the person to put you in touch with.”
“There is an interesting Spanish connection,” says Nogales. “I don’t work with amyloids, but because I work on Tau protein when it is bound to microtubules, I know about the fibrillary tangles that Tau makes. Because of that, I know Sjors Scheres, PhD, of the MRC Laboratory of Molecular Biology in Cambridge, UK, and his work on Tau amyloids in Alzheimer’s disease.” Nogales adds that she knew Scheres from his time at the National Center for Biotechnology in Madrid, where she often gives seminars while she’s there visiting friends. “He speaks Spanish better than me,” she laughs.
“Scheres developed a program that most of the cryo-EM community uses to process the raw data that comes out of the machine into the 3D density maps,” says Fitzpatrick. “From those maps, we build the atomic-scale models. He’s like the expert in amyloid helical reconstruction.”
“Sjors is an absolute star. Lots of people want to collaborate with him,” says Nogales, “but he can only spread himself so thin. In this case, I’m talking to Ruben, and I’m from Spain, and he’s also from Spain, and we have this special connection. Plus, the project is super cool. It’s easy to get excited about this work.”
“I told him, because Sjors works on amyloids, he will know exactly how to process the samples. So I put them in touch, and then really quickly, they had determined the structure.”
Being their matchmaker, Nogales says, “I actually found out before anyone else. They were complementary and they work well together. It’s a happy story—I wish it worked out like this all the time!”
Kudos goes to Hervas, who had to screen hundreds of buffer solutions, as well as isolate a huge amount of ultrapure Orb2 protein. “Ruben extracted protein from three million fruit fly heads. If that doesn’t deserve some kind of award, I don’t know what does!” exclaims Fitzpatrick.
“One of the things I deal with all the time is, for lack of a better phrase, ‘crap in, crap out’—so if you have a bad sample, you’re going to get bad data,” says Fitzpatrick. That certainly was not the case here. “One of the reasons they had such a high-resolution structure is because the quality of the sample going in was just so pure, it was easy to track the fibrils.”

The Si Lab uses the fruit fly system to study biological aspects of memory persistence.
“This is an example of people doing work that opens the mind to think of things in a new way,” says Nogales. “I’m not a neurobiologist, but memories last much longer than the “lifetimes” of individual proteins. At a cellular and molecular level, how can that be?”
This work provides one possible solution to that question: That the form of Orb2 required for memory persistence is an amyloid, and that Orb2 amyloid has such slow turnover as well as the capacity to incorporate newly generated protein into that form, “not as a way of taking away functionality, but as a way of creating functionality—that’s what I think is cool,” says Nogales. “It’s not a pathological state, it’s just a functional state.”
Another important question is—if amyloids are so stable under physiological conditions, how can memory be dynamic? One potential clue can be found in the central core of the amyloid structure. Unlike the hydrophobic, or water-repelling, core of pathogenic amyloids, the hydrophilic, or water-loving, core of Orb2 amyloids suggests how some neuronal amyloids could be a stable yet regulatable substrate of memory. The hydrophilic surfaces of the Orb2 amyloid fold could be susceptible to modification in a way that’s fundamentally different than hydrophobic core amyloids. “From a structural point of view, that is also very cool,” says Nogales.
What’s next
The exploration of functional amyloids in neurobiology has only just begun. “Studying this problem has led us to areas of study that I never thought I’d be working in. Now we study gene splicing and protein chaperones, as well as how many shapes a protein can take and how.” The Si Lab is also studying the molecular basis of forgetting. That is, does the dissolution of Orb2 amyloid dictate when a memory disappears? If so, does it just disintegrate, or is that process also tightly regulated?
Among many other projects, the Si Lab is now working on a mouse model to test whether CPEB is required for long-term memory, and whether it also self-aggregates as an amyloid.
“The key question is moving this line of research from fruit flies to mice to humans,” says Si. Although the possibility exists that something in the vertebrate or mammalian nervous system doesn’t allow the use of functional amyloids as a mechanism of information storage, “it would be very hard to imagine that this process of memory, which has evolved to be utilized by so many biological organisms, is suddenly not utilized in humans.”
Once again, proving it will not be trivial.
“The challenge is whether the methods that they developed for working in fruit flies—for extracting the purified protein—will be easily applied to human biopsy samples,” says Fitzpatrick. “We have a lot of the data processing hurdles sorted out. I think once we get a good sample, we’ll be able to generate data pretty quickly.”
“Ultimately, it’s science,” says Si. “It’s not what we think, it’s what is. We’ll keep working to figure out what’s going on.”
News

09 October 2025
An annual tradition marks the start of scientific discovery for the 2024-2025 class of Stowers Graduate School students.
Read Article
In The News

03 July 2024
From In Kansas City Magazine: Meet 10 people, including Stowers Scientific Director Kausik Si, Ph.D., who are making a difference in Kansas City.
Read Article
In The News
08 March 2024
From KSHB, one of the area's top scientists, Kausik Si, Ph.D., from the Stowers Institute received a coveted award for his "paradigm shifting" work to understand how our memory works and how that defines us.
Read Article
Press Release
21 February 2024
Scientific Director Kausik Si from the Stowers Institute for Medical Research alongside Investigator Lukasz Joachimiak from the University of Texas Southwestern Medical Center received CZI's Collaborative Pairs Pilot Project Awards grant for their project titled, “Tuning memory by altering amyloids.”
Read Article