News

14 June 2022
Stowers welcomes 2022 ASBMB PROLAB winner to the Bazzini Lab
Pallarés, a PhD student, will arrive at the Stowers Institute in July after being named a 2022 PROLAB winner.
Read Article
News
New research examines the critical transition in development common to all animals—when a zygote switches from using maternally-supplied RNA to the point in time it can transcribe its own RNA.
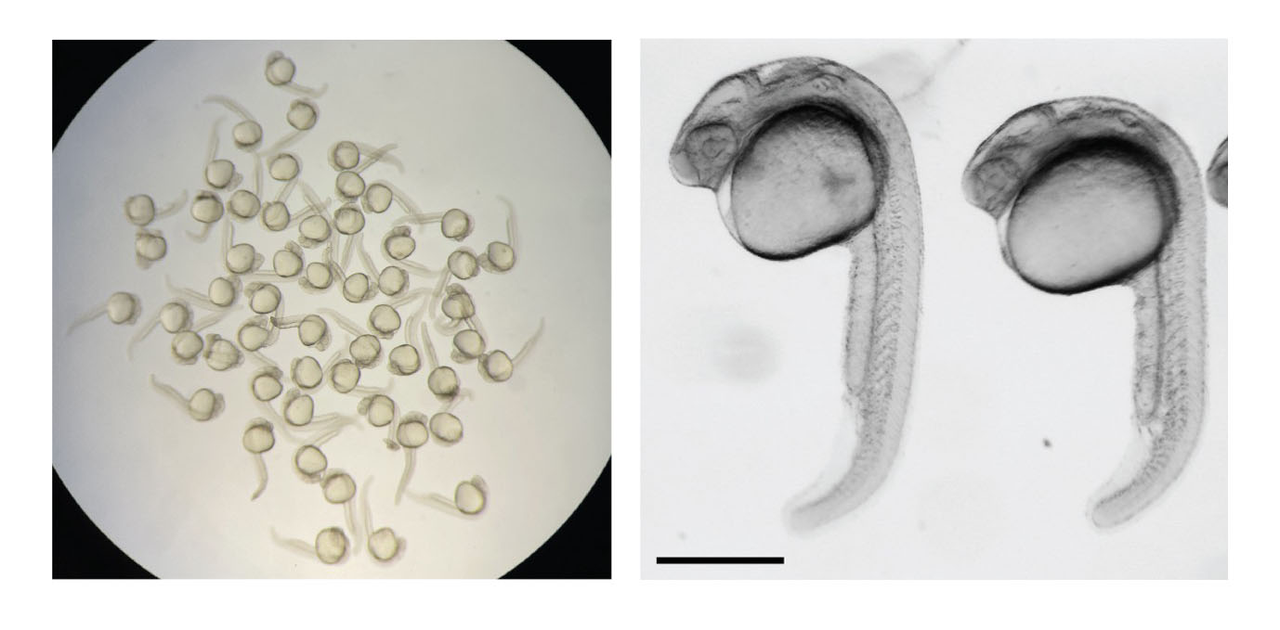
Developing zebrafish embryos.
By Rachel Scanza, Ph.D.
All animals that begin as a single fertilized cell, humans included, need a helping hand from mom to jumpstart their genomes.
Egg cells are packed with all the molecules that a zygote—the little ball of cells marking the earliest embryonic stage—needs to begin its development. Using the material provided by the egg, the zygote must also begin manufacturing its own RNA molecules from its newly formed genome, some of which instruct the assembly of proteins, helping to establish identity and function of all the cells it will need to grow into an adult.
New research from the Stowers Institute for Medical Research examined this critical transition in development common to all animals—when a zygote switches from using maternally-supplied RNA to the point in time it can transcribe its own RNA. Published in Genome Biology on March 19, 2024, the study led by Stowers Graduate School Predoctoral Researcher Danielson Baia Amaral from the lab of Associate Investigator Ariel Bazzini, Ph.D., is shedding new light on mechanisms guiding this process known as the maternal to zygotic transition, and the corresponding activation of the zygote’s genome in zebrafish.
“The embryonic stage right after fertilization involves a major change,” said Baia Amaral. “This change involves the systematic degradation, or breakdown, of most RNA molecules provided by the egg.”
As the developing animal starts synthesizing its own DNA messages and proteins, it needs to get rid of the material provided by mom. Maternal RNAs are degraded by several mechanisms. The authors focused on one involving microRNAs, which also act more broadly to regulate gene activity throughout development, in adult organisms, and in certain diseases like cancer.
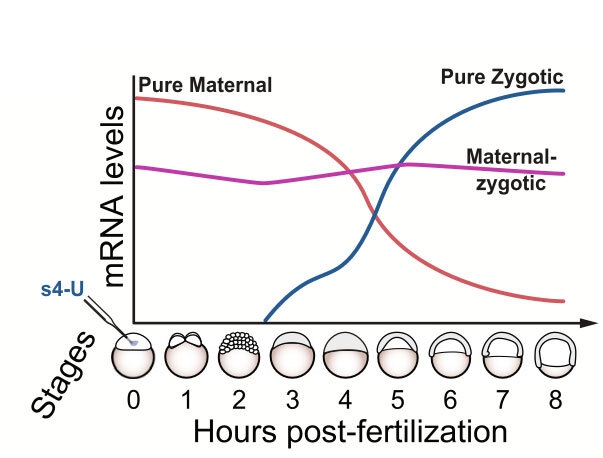
Graphical representation of mRNA levels over the first eight hours of zebrafish development.
MicroRNAs are very small RNA molecules that generally help a cell control the kinds and amounts of proteins it needs. They do not encode proteins. Instead, they act as tiny probes that instruct the degradation and silencing of protein encoding RNAs (mRNAs) by binding to target sequences within the mRNA. MicroRNAs essentially act like genetic scissors, altering or destroying their targets similar to how CRISPR technology works.
“We knew that microRNAs are actively degrading maternal mRNAs but wanted to assess the extent to which these also degrade new mRNA made by the zygote,” said Baia Amaral. The advent of an RNA sequencing technique called SLAM-seq, which adds a molecular label to newly made RNA, enabled the team to differentiate between the maternal RNA and new zygotic RNA.
“Beyond eliminating maternally inherited mRNA, our findings suggest that microRNAs are also fine-tuning the expression of messages made by the embryo,” said Baia Amaral.
Since microRNAs are regulating both inherited and freshly made mRNA, and because the same processes occurring in zebrafish embryos are at play in many other species including humans, this research is giving rise to new questions.
“Our results show that the embryo is making new copies of the maternally provided RNAs it is actively degrading, leading us to ponder why the embryo is creating new things it is trying to get rid of,” said Baia Amaral. “In addition, microRNAs are implicated in many human conditions, causing us to wonder if the same phenomena we are observing in development may also be at play in disease.”
Additional authors include Rhonda Egidy and Anoja Perera.
This research was supported by the National Institutes of Health (NIH) (awards: R01GM13849, R21OD034161) and institutional support from the Stowers Institute for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
News

14 June 2022
Pallarés, a PhD student, will arrive at the Stowers Institute in July after being named a 2022 PROLAB winner.
Read Article
Press Release
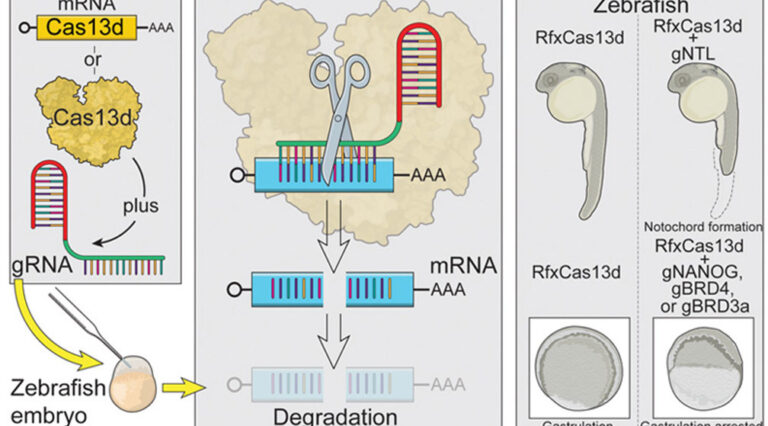
07 August 2020
Scientists in the Bazzini Lab and collaborators have harnessed CRISPR technology to target gene messages in animal model embryos to gain a better understanding of the early stages of vertebrate development.
Read Article
News
18 November 2025
Stowers Associate Investigator Ariel Bazzini, Ph.D., discusses a collaboration that uncovered a new mechanism guiding the earliest steps of life.
Read Article