News
03 October 2025
How one gene flips the fate of sensory cells
Stowers scientists discover a master gene in zebrafish that determines hair cell type, a potential target for restoring hearing loss in humans
Read Article
Press Release
New research used the highly regenerative zebrafish to investigate the timing and genetic programs of macrophages in the repair and regeneration of a zebrafish sensory organ.
Is there a connection between the immunesystem and regeneration? New research from Nicolas Denans, Piotrowski Lab, published in Nature Communications, found that macrophages are required for organ regeneration in a regenerating species. Listen as Investigator Tatjana Piotrowski and Postdoctoral Researcher Nicholas Denans explain their findings in-depth. Video courtesy of the Piotrowski Lab, Stowers Institute for Medical Research.
KANSAS CITY, MO — How the immune system responds to injury in many organs and tissues allows and enables their repair and regeneration. Yet for some species like humans, damage to organs such as the brain, spinal cord, or heart is irreversible. Imagine if we were able to regenerate these. For organ transplant candidates and recipients, the nerve-wracking wait for “the call,” or the lifelong need for immunosuppressing medications would no longer be necessary.
New research from the Stowers Institute for Medical Research used the highly regenerative zebrafish to investigate the timing and genetic programs of macrophages, a type of white blood cell, in the repair and regeneration of a zebrafish sensory organ. Understanding how the immune system responds to injury, first by inducing inflammation immediately followed by an anti-inflammatory response, provides invaluable knowledge for designing targeted immunotherapies that may be applicable in combatting human conditions like hearing loss or deafness, heart or spinal cord damage.
Recently published in Nature Communications on September 20, Postdoctoral Researcher Nicolas Denans, PhD, in the lab of Stowers Investigator Tatjana Piotrowski, PhD, discovered a new macrophage anti-inflammatory paradigm. Rather than the established view that anti-inflammatory activation states for macrophages are linked to just one type of signaling pathway, Denans found that the same population can, and must, transition through each of three anti-inflammatory states for organ regeneration.
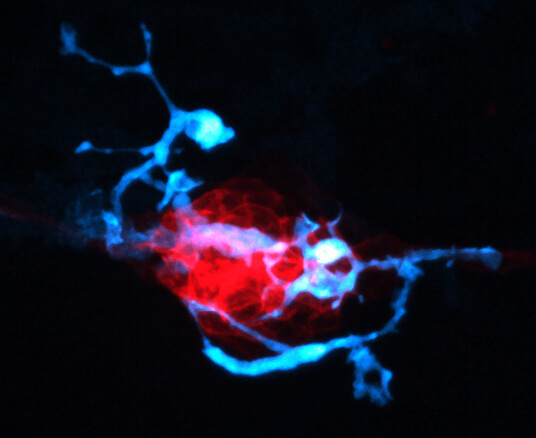
Three hours after antibiotic treatment, macrophages (blue) invade the zebrafish sensory organ (red), engulfing and digesting dead hair cells and debris.
“Organ regeneration offers an exciting opportunity for studying the immune system and to inquire why some species can regenerate organs like the heart or missing limbs while others like humans cannot,” said Piotrowski.
Zebrafish sensory organ hair cells are an ideal system to investigate the pathways and cell types involved in regeneration since they are easily destroyed with antibiotics and begin regenerating within five hours. This enabled the researchers to identify the exact timing and genetic programs for each anti-inflammatory macrophage activation state.
“Our hypothesis is that human macrophages do not receive the proper chemical activation “cocktail” to instruct pro-regenerative processes,” said Denans. “Identifying the molecular recipe of macrophage activation in zebrafish may one day enable us to design regenerative immunotherapies in humans.”
Essential for organ regeneration, macrophages, which in Latin literally translates as “big eaters,” engulf foreign particles like dead cells and bacteria and use enzymes to digest them. In addition to their culinary appetite, these cells signal both pro- and anti-inflammatory pathways to secrete chemicals, or cytokines to either recruit additional types of white blood cells or trigger anti-inflammatory pathways for cellular and tissue repair.
Investigating macrophages at high spatial resolution and at multiple closely spaced time points during zebrafish sensory hair cell death and regeneration was critical. For the first time, the study demonstrates that a single population of this cell type sequentially and independently transitions through three different anti-inflammatory states, each with its own unique molecular and genetic signature.
“The new evidence is a valuable resource for comparative studies on the genetic programs involved in macrophage-mediated repair and regeneration,” said Denans. “In other words, different types of injuries may induce different kinds of inflammatory responses. We want to decipher whether this “language” is universal or if there are a variety of dialects.”
While the study marks the first time that the sequential macrophage states have been resolved with extraordinary precision, preliminary comparisons with previously reported pathways in different organs and species suggest that this mechanism is likely conserved.
News

03 October 2025
Stowers scientists discover a master gene in zebrafish that determines hair cell type, a potential target for restoring hearing loss in humans
Read Article
News

26 September 2025
"The real-world experience of being in a lab will be beneficial in helping you determine if you want a career in science."
Read Article
In The News

25 July 2025
Published in Technology Networks, scientists have discovered how two genes control the regeneration of sensory hair cells in zebrafish, offering new clues for addressing hearing loss in humans. Published in Nature Communications, the study, led by Tatjana Piotrowski, PhD, provides a clearer picture of how stem cells and their progeny divide to replenish damaged tissue – a process that fish perform naturally but humans cannot. These findings could open new avenues for regenerative medicine research targeting hearing and balance disorders.
Read Article