Research
Research
We pursue four, tightly connected themes across diverse protein systems and branches of the tree of life.
How do proteins evolve to fold?
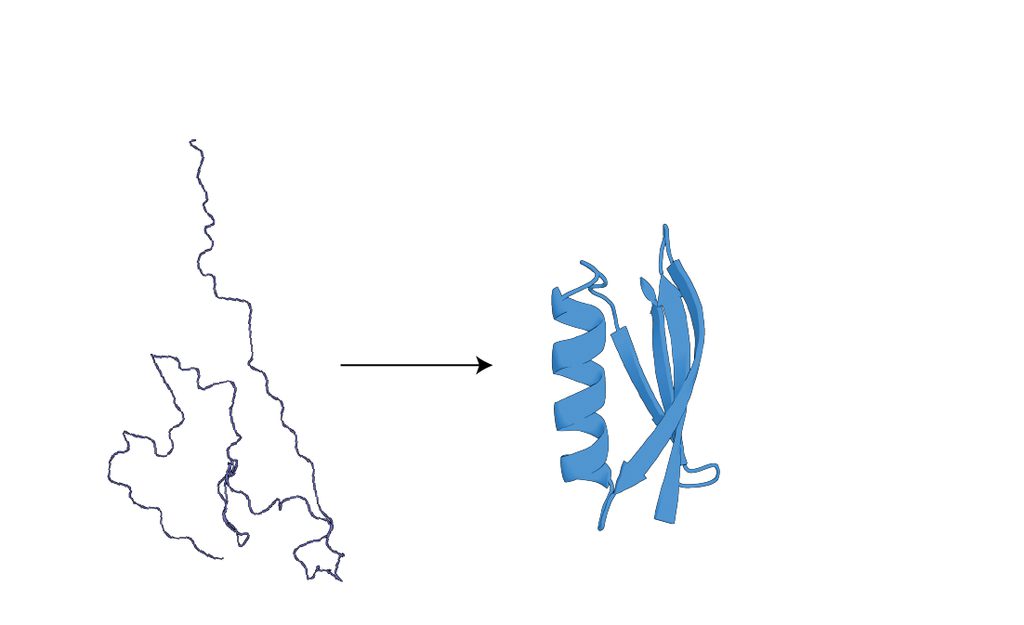
In order to function, most proteins must fold into precise three dimensional structures held together by many interacting residues. While a relatively small set of folds accounts for most known structures, the paths by which new folds originate remain obscure. We aim to explore this process by identifying and reconstructing protein histories wherein folds arose from “scratch”, from silk-forming proteins in Spiders and Moths to the many independent evolutions of carbonic anhydrases across bacteria, to the evolution of entirely new viral folds by overprinting on previously existing genes.
How do proteins evolve to assemble into higher-order complexes?
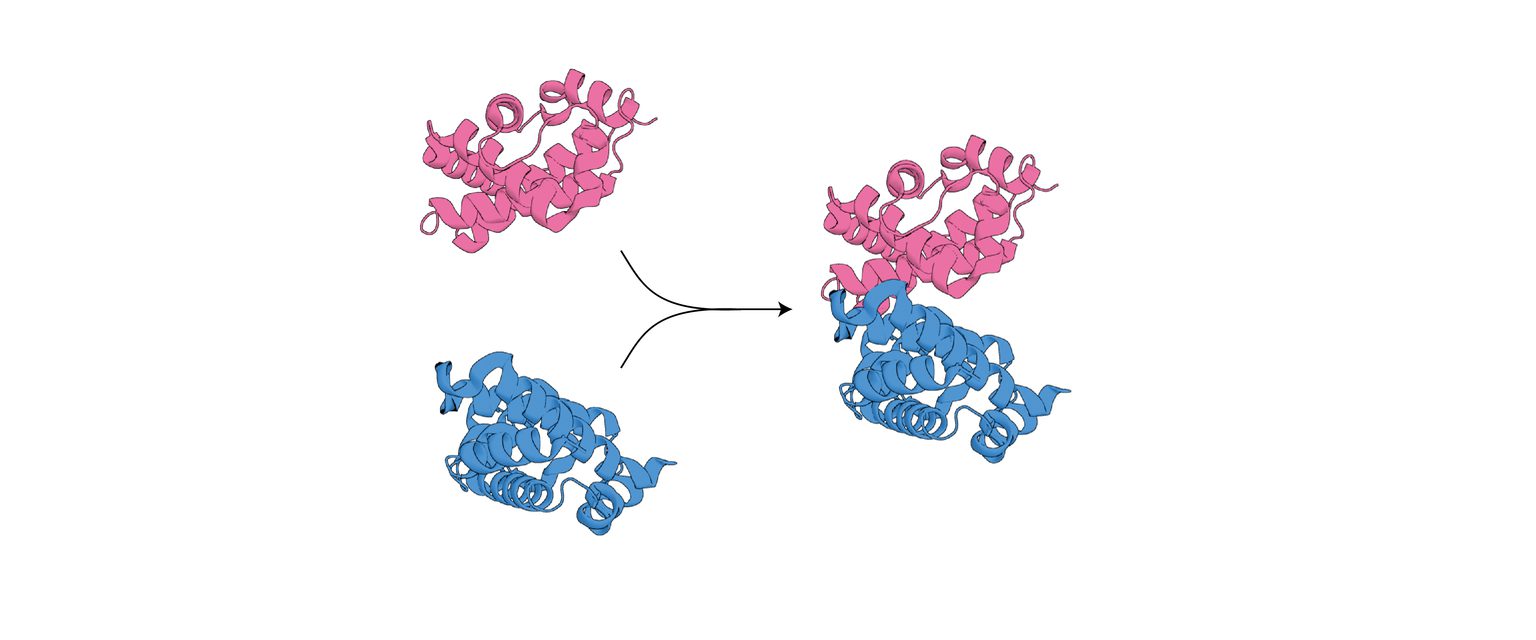
Rather than functioning as isolated molecular units, proteins assemble into higher-order structures composed of multiple components. These assemblies are highly specific and involve interfaces with tight electrostatic complementarity and steric fit. We aim to discover how and why nature builds these complicated interfaces, including the sub-nanomolar, high-affinity interfaces exhibited by the scavengers and chaperones involved in hemoglobin metabolism, which evolved early in vertebrate history by recruiting proteins or domains that were previously monomeric.
Catalysis
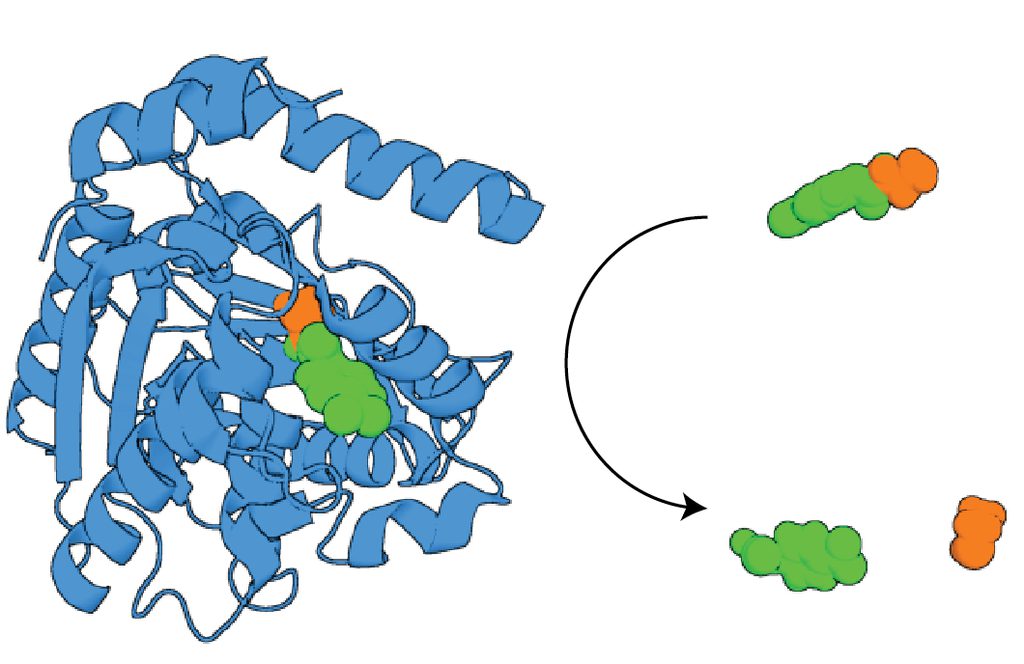
Among the most remarkable aspects of protein biology is the ability of enzymes to dramatically accelerate chemical reactions, enabling life to make, break, and rearrange bonds that power metabolism. This action arises from the precise placement of amino acids and functional groups in 3D space within a protein’s fold, facilitating the electron and proton transfers (and the electrostatic environments) needed for productive chemistry. We are interested in how proteins evolve to be catalytic. To explore this question, we use the lipocalins - a class of small-molecule binding proteins found across the animal kingdom - to examine historical instances where a catalytic function emerged from simple binding (e.g., lipocalin-type prostaglandin D synthase) and to assess the evolutionary accessibility of new enzyme functions. We are also interested in the extent to which the enzyme functions of modern proteins can be encoded by structurally simpler analogs, which has implications for their accessibility from random sequence space.
Design of Allosteric machines
Proteins are not static molecules but rather toggle between different structural conformations. This allows them to respond to signals and convert chemical energy into work. Creating proteins with precisely tuned dynamical properties - such as those governing cellular receptors and protein motors - is a major frontier for protein design. We strive to engineer new and useful allosteric dynamics into natural proteins, with the aim of creating fuel-driven molecular machines and biosensing platforms. We are aided by a panoply of deep-learning-based computational tools that allow us to model, predict and specify conformational change. The engineering principles uncovered will also illuminate the biophysical factors that shape the evolutionary origins of allostery. On this front we aim to control enzyme function allosterically with synthetically designed proteins, and to engineer de novo disaggregases that can pull apart mechanically stable assemblies.