Lab Fun

27 October 2025
Pumpkin Carving
Fall fun!
Read Article
News
Collaboration with Stowers scientists uncovers how cells cope with defective ribosomes
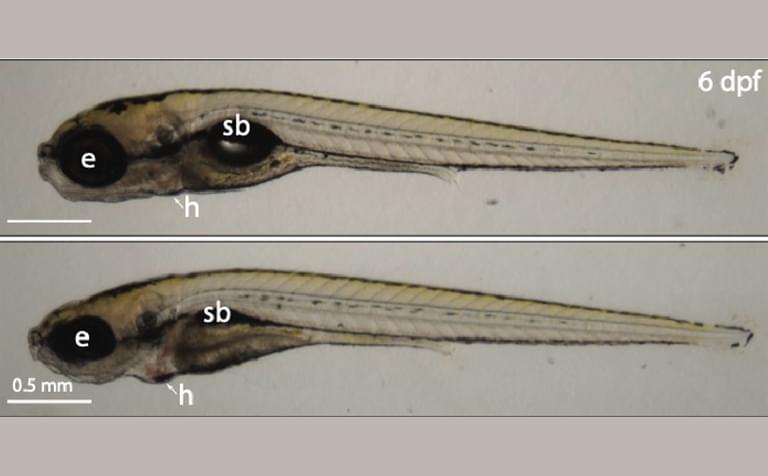
Image of control (top) versus znf574 mutant zebrafish (bottom) at six days post fertilization show that defects in the Ribosome Assembly Surveillance Pathway cause ribosomopathy-like developmental disruptions. Highlighted are the eye (e), swim bladder (sb), and the heart (h).
By Rachel Scanza, Ph.D.
A team of researchers, including scientists at the Stowers Institute for Medical Research, discovered how organisms monitor the health of ribosomes, the molecular machines that make proteins in all living organisms. New research uncovered a key protein involved in detecting faulty ribosomes and shed light on how failure to clear such ribosomes can lead to disease.
Proteins, which are critical for all cellular function, are assembled by ribosomes. Even ribosome production requires proteins, and assembly is a highly complex process prone to inevitable errors. While scientists have known that ribosomes get broken down when they are made incorrectly, it is still unclear how cells know which ones to discard.
“Our cells build thousands of ribosomes every day,” said Stowers Assistant Investigator Kamena Kostova, Ph.D. “This assembly process is susceptible to environmental factors. If something goes wrong along the way, faulty ribosomes can form, which may be harmful to the cell.”
Now, a study published in Molecular Cell on May 5, 2025, discovered a protein called ZNF574 as a critical component within a newly identified ribosome quality control pathway. Co-led by Carnegie Institution for Science laboratory technicians Jared Akers and Michael LaScola, along with Kamena Kostova, Ph.D., the team determined that ZNF574 is a part of the pathway they termed the Ribosome Assembly Surveillance Pathway and found that loss of ZNF574 function in zebrafish led to defects in development.
The researchers developed a method to pause the maturation of faulty ribosomes in human cells, tagging them with fluorescent proteins to visualize their fate. The team could clearly see that defective “pre-ribosomes” in the process of assembly were quickly destroyed.
Cartoon illustrating how the Ribosome Assembly Surveillance Pathway detects faulty ribosomes for degradation. Credit: Caitlin Rausch, Warbler Creative.
Next, the team screened for factors responsible for the observed degradation, identifying the gene, ZNF574 as a key player in the surveillance pathway. Additionally, the researchers observed that depleting ZNF574 resulted in the accumulation of defective ribosomes, which in turn prevented new ribosomes from assembling.
“We developed a new system to intentionally create faulty ribosomes in human cells, allowing us to investigate how the cell detects and removes these defective parts,” said Kostova. “We discovered a previously unknown quality control system, which helps identify and send faulty ribosomes to the cell's ‘recycling center’.”
Defects in ribosome production can cause a group of disorders collectively called ribosomopathies, which are characterized by anemia and craniofacial abnormalities. Zebrafish are vertebrate research organisms frequently used to study ribosomopathies. The team discovered that loss of ZNF574 and therefore accumulation of defective ribosomes causes ribosomopathy-like abnormalities in zebrafish.
“Importantly, we found that when this pathway is broken—in both human cells and a zebrafish model—faulty ribosomes build up, leading to problems with cell function and development,” said Kostova.
Ribosome assembly is a fundamental process across the tree of life. Uncovering the Ribosome Assembly Surveillance Pathway quality control mechanism and the protein that governs it may enhance our understanding of both normal development and disease. The new findings may be significant for uncovering novel treatments to address conditions caused by defects in ribosome formation.
Additional authors include Adrian Bothe, Hanna Suh, Carmen Jung, Zachary D. Stolp, Ph.D., Tanushree Ghosh, Ph.D., Liewei L. Yan, Ph.D., Yuming Wang, Michelle Macurak, Amisha Devan, Mary C. McKinney, Ph.D., Tarabryn S. Grismer, Andres V. Reyes, Eric J. Ross, Tianyi Hu, Shou-Ling Xu, Ph.D., and Nenad Ban, Ph.D.
This work was funded by National Institutes of Health (NIH) Directors’ Early Independence Award (award: 5DP5OD028147-03), the Carnegie Endowment Fund, the Jane Coffin Childs Postdoctoral Fellowship, the National Institute of General Medical Sciences of the NIH (awards: F32GM143875, R01GM135706), the Boehringer Ingelheim Fonds Ph.D. fellowship, the Swiss National Science Foundation (SNSF) (award: 310030_212308), the National Center of Excellence in Research RNA and Disease Program of the SNSF (award: 51NF40-205601), and with institutional support from the Stowers Institute for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.